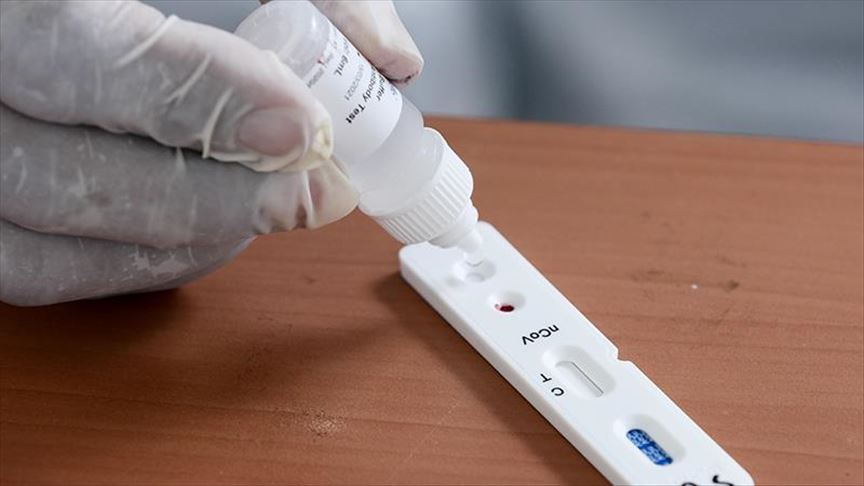
Indian Medical Council has recently ordered the states who are using Rapid Testing Kits from China to stop the testing procedure as these kits have found to deliver inappropriate results. What are Rapid testing kits? How does it find in identifying the virus presence in the body and why are those kits from China found faulty. Lets us know about them through this article.
RAPID TESTING KITS
- Indian Medical council recently suggested the states who are using Rapid Testing Kits to stop the testing procedure and try RT-PCR test which is a way better than Rapid Testing especially that were imported from China.
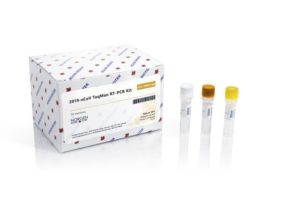
- RT-PCR (Reverse Transcription – Polymerase Chain Reaction). This method is used to identify the presence of specific genetic material from any pathogen, including a virus.
- They first convert the RNA into DNA. This is because only DNA can be copied and further amplified.
- The DNA is amplified thousands of times to search for the virus which is easy to find than to find a minuscule amount of virus.
WHY NOT RAPID TESTING KITS:
- Rapid Testing Kits provided inappropriate results in the UK and the USA. According to the statistics released by the UK government, nearly 30% positive cases tested negative using the Rapid Testing Kits.
- This is because those kits are less sensitive to the anti-bodies.
- The same response was shared between countries like the UK, the USA, Spain, and now India.
One main reason for the failure of Rapid Testing Kits is that China government gave permissions to some small firms which do not have enough experience in manufacturing them. Thus test outcomes from these kits should not be taken as criteria to conclude that a person is corona free. It is found that China is exporting kits to all most all the European Countries where more than 100 companies are manufacturing them.
Some reports stated that the companies that are manufacturing kits in China do not have a proper license. According to the European agency, a Rapid Testing Kit is to be approved for use only when it shows 80% accuracy. But, the kits in Spain, the UK, the USA, and India showed only 30% accuracy.

Not only the kits from China, ICMR has identified that the kits approved by the US Food and Drug Administration are also found faulty. Those kits imported from the USA were used for monitoring and surveillance. But, ICMR stated that those kits even got failed in surveillance. The Indian government, ICMR, and the DRDO are working closely with each other to develop an Indian made Rapid Testing and RT-PCR testing kits under Make In India Initiative.
-KRISHNA
EDITOR IN CHIEF, FrontlinesMedia