Quality Systems Manager—Regulatory & Strategic Focus
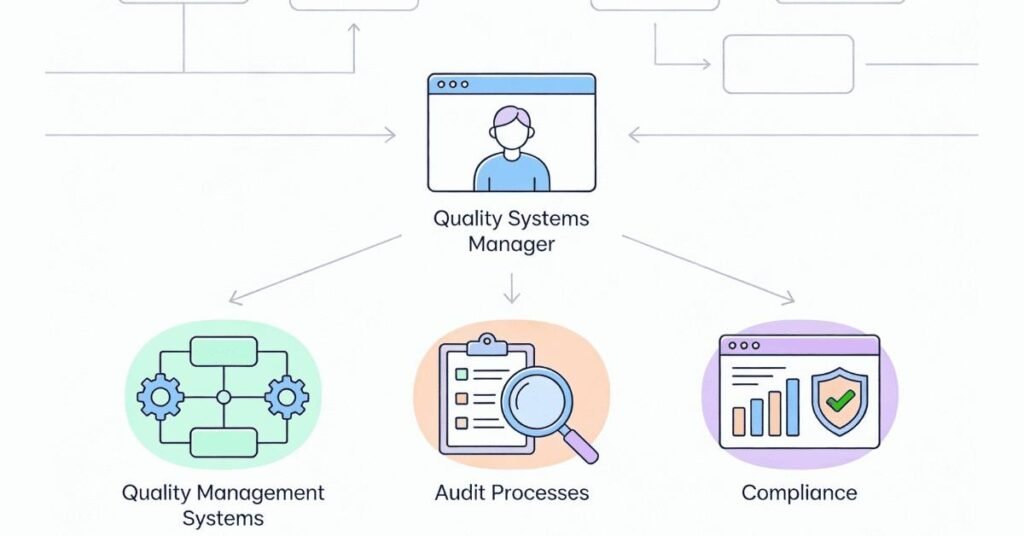
Table of Contents
The Compliance Guardian: Quality Systems Manager
Quality systems managers ensure entire quality infrastructure works—systems, procedures, compliance, audits, documentation. They earn ₹65,000-1,00,000/month, advancing to ₹1,20,000-1,90,000+/month as directors.

What Quality Systems Managers Do
Systems Management (35%):
- Develop quality management systems (ISO 9001, IATF, pharma-specific)
- Create and maintain procedures
- Ensure system compliance
- Lead internal audits
- Prepare for external audits
- Manage quality documentation
Regulatory Compliance (30%):
- Ensure regulatory compliance (OSHA, FDA, environmental laws)
- Stay current on changing regulations
- Implement compliance requirements
- Prepare for regulatory inspections
- Manage compliance documentation
- Handle recalls/non-conformances
Supplier Quality Management (20%):
- Develop supplier quality standards
- Audit suppliers
- Monitor supplier performance
- Manage supplier issues
- Implement supplier development programs
- Handle supplier improvements
Strategic Quality (15%):
- Plan quality strategy
- Identify system improvements
- Manage quality risks
- Support business growth
- Influence product design quality
Technical Expertise Required
Quality Management System Knowledge:
- ISO 9001 and industry-specific standards
- IATF 16949 (automotive)
- FDA cGMP (pharma)
- ISO 13485 (medical devices)
- AS9100 (aerospace)
Regulatory Knowledge:
- OSHA standards
- EPA environmental requirements
- Industry-specific regulations
- International standards (CE marking, RoHS, etc.)
Audit & Assessment Skills:
- Internal audit techniques
- Audit report writing
- Non-conformance management
- Corrective action systems
- Risk-based thinking
Documentation & Control:
- Quality manual development
- Procedure documentation
- Control of documents
- Record management
- Traceability systems
Salary Expectations
Quality Systems Specialist:
₹50,000 – ₹70,000/month
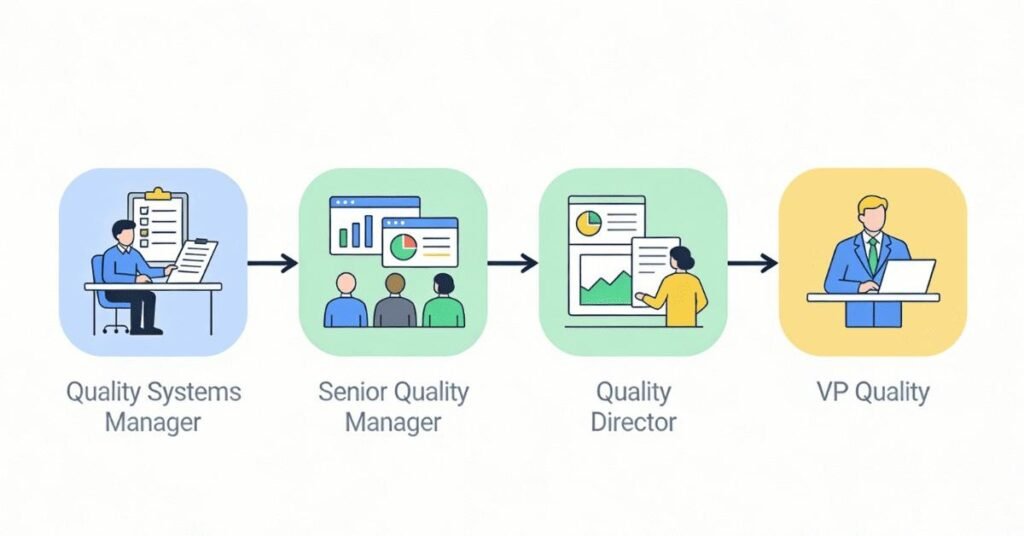
Quality Systems Manager:
₹70,000 – ₹1,05,000/month
Senior Manager / Quality Systems Director:
₹1,05,000 – ₹1,60,000/month
Quality Director:
₹1,60,000 – ₹2,40,000+/month
Entry Requirements
- 8-10 years quality experience
- ISO audit/internal audit experience
- Quality procedure development
- Strong regulatory knowledge
Strategic thinking capability
The Bottom Line
Quality systems managers ensure company compliance and quality infrastructure. Entry ₹70,000-1,05,000 → 5-year ₹1,05,000-1,60,000+. Strategically important role. Regulatory expertise highly valued.
